DCS - Computer System Validation
DCS - Distributed control System
A risk-based framework for validating and managing computerized systems in GxP-regulated environments, especially in the pharmaceutical and medical device industries, is offered by the International Society for Pharmaceutical Engineering (ISPE) guidelines known as GAMP-5 (Good Automated Manufacturing Practice 5). Manufacturers can make sure their systems are dependable, efficient, and up to quality standards by following this collection of best practices, which is not a law in and of itself.
Agile Methodology. In 2008, the first edition of GAMP 5 proposed a linear methodology for software development and validation. Second Edition, the newest version of the ISPE's computerized system guidance, was released in July 2022
The GAMP5 approach is the foundation of DCS Validation. This method of validating a computerized system or control system is risk-based as a manual for computer system validation (CSV).
21CFR Part 11 is playing a key role in pharma Data integrity which need to be complying with ICH Q7
RACI Matrix for DCS Validation Deliverables as per GAMP-5 ( CSV )
A key role in the DCS validation process is played by the RACI matrix.
The principal vendor will be held accountable and in charge of the entire project, including the control philosophy and architecture.
Principal Vendor to guarantee that engineering, paperwork, installation, and other aspects adhere to global standards.
The table that follows outlines roles and duties for carrying out the project as per V-Bird method validation flow.
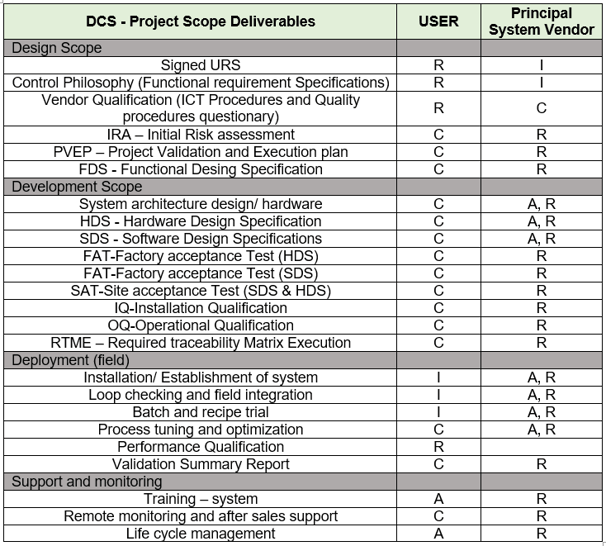
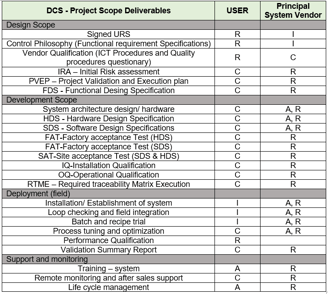
URS: User requirement specifications, which consists of User expectations from the DCS like Operations, Functions, security, monitoring , controlling, user interface, interlocking system graphical interface, etc.,
FRS: Functional Requirement specifications, it describes the Process controls philosophy and flow of a process.
Vendor Qualifications : OEM - Original Equipment (product) Manufacturer.
Initial Risk Assessment : Identify potential risks in your control system using the FMEA (Failure Mode and Effects Analysis) tool. This process helps in assessing vulnerabilities, reducing risks, and implementing effective mitigation strategies.
PVEP : Project validation and execution plan, Outlines the methodology for validating the Control System or Distributed Control System (DCS), detailing the steps and criteria for successful execution.
Functional Design Specifications: Defines how the control system will operate, as developed by the Original Equipment Manufacturer (OEM), tailored specifically to the requirements of the project.
HDS : Hardware design specifications, it consists of control system architecture, panel wiring drawings, schematic drawings, loop drawings of a control system or a DCS etc. It also encompasses detailed specifications and datasheets for every component used.
SDS : software design specification, control philosophy and each operation coding and development process will be explored by an OEM.
FAT : Factory acceptance test, this will be performed at OEM factory premises for the Hardware or software before dispatching from the factor. It helps identify gaps and areas for improvement, significantly reducing the risk of failures at the end-user site.
What is 21 CFR Part-11 in Pharma :
The United States Food and Drug Administration's (FDA) rules for electronic records and electronic signatures (ERES) are outlined in Title 21 CFR Part 11 of the Code of Federal Regulations.
Difference between GAMP-5 & 21 CFR Part-11 : 21 CFR Part 11 focuses on electronic records and signatures, while GAMP 5 focuses on validating automated systems
In regulated businesses, 21 CFR Part 11 provides a framework for guaranteeing the security, integrity, and traceability of electronic documents. This rule makes it possible for the regulated businesses to modernize through simplified, safe, and effective data handling.
Key Factors for 21 CFR Part-11:
User management
Alarms, Events, Audit trails and reporting system.
Digital Signature and Traceability.
User Management Recommendations :
User and group should be controlled centrally usually Domain & Active directory.
Password policies to be established.
a) Password expiry to be defined. usually 5 min/10min/15min.
b) Unique User ID and strong password ( password complexity).
